Symptoms of Allergic Conjunctivitis
The symptoms of allergic conjunctivitis – ocular itching and tearing – are chronic, painful and persistent, affecting the quality of life and leading to a loss of work that can create a substantial economic burden on patients and their families. In fact, allergic conjunctivitis is one of the most common diseases treated by ophthalmologists. In many cases, physicians and patients say currently available therapy is inadequate.
Approximately 100 million patients in the United States have allergic conjunctivitis, and we estimate that up to 30 million of these patients either don’t respond adequately to, or are dissatisfied with, topical antihistamines, the current standard of care. The reason: antihistamines appear to lack durable activity, which may be because histamine is only one of the biological mediators of allergic conjunctivitis and the fact that increased histamine levels persist for only up to 20 minutes after allergen exposure.
Because many patients have symptoms of both dry eye disease and allergic conjunctivitis differential diagnosis can be challenging for physicians. Approximately half of dry eye patients complain of itching, which is generally considered the result of an allergy, while approximately half of the allergic conjunctivitis patients complain of dryness, which is generally considered the result of dry eye disease.
There are no U.S. Food and Drug Administration (FDA)-approved therapies indicated to treat both dry eye disease and allergic conjunctivitis. Neither cyclosporine nor lifitegrast have been approved for use in patients with allergic conjunctivitis, and antihistamines are known to exacerbate ocular dryness. Thus, with the possible exception of topical corticosteroids, which cause glaucoma and other serious ocular toxicities in some patients, we believe that no currently available drug for dry eye disease or allergic conjunctivitis is likely to be effective for the treatment of patients who experience symptoms of both diseases.
There are approximately 100 million patients in the United States with allergic conjunctivitis.
Reproxalap: Our Novel Small Molecule Drug Candidate for Allergic Conjunctivitis
By inhibiting RASP, which are elevated in a variety of inflammatory diseases, reproxalap represents a novel mechanism for diminishing ocular inflammation in allergic conjunctivitis. In clinical trials, reproxalap has consistently demonstrated statistically significant and clinically relevant activity. We believe that reproxalap may have a more advantageous therapeutic profile than currently approved drugs for each indication, having shown the potential for early durable activity in allergic conjunctivitis. Additionally, reproxalap, if approved, also has the added potential of being the only product that may be able to effectively treat allergic conjunctivitis, uniquely addressing the needs of the large underserved population that suffers from this disease. Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns reported. Mild instillation site irritation is the most common adverse event reported.
Aldeyra intends to advance 0.25% topical ocular reproxalap, our lead RASP inhibitor, for the treatment of dry eye disease and allergic conjunctivitis.
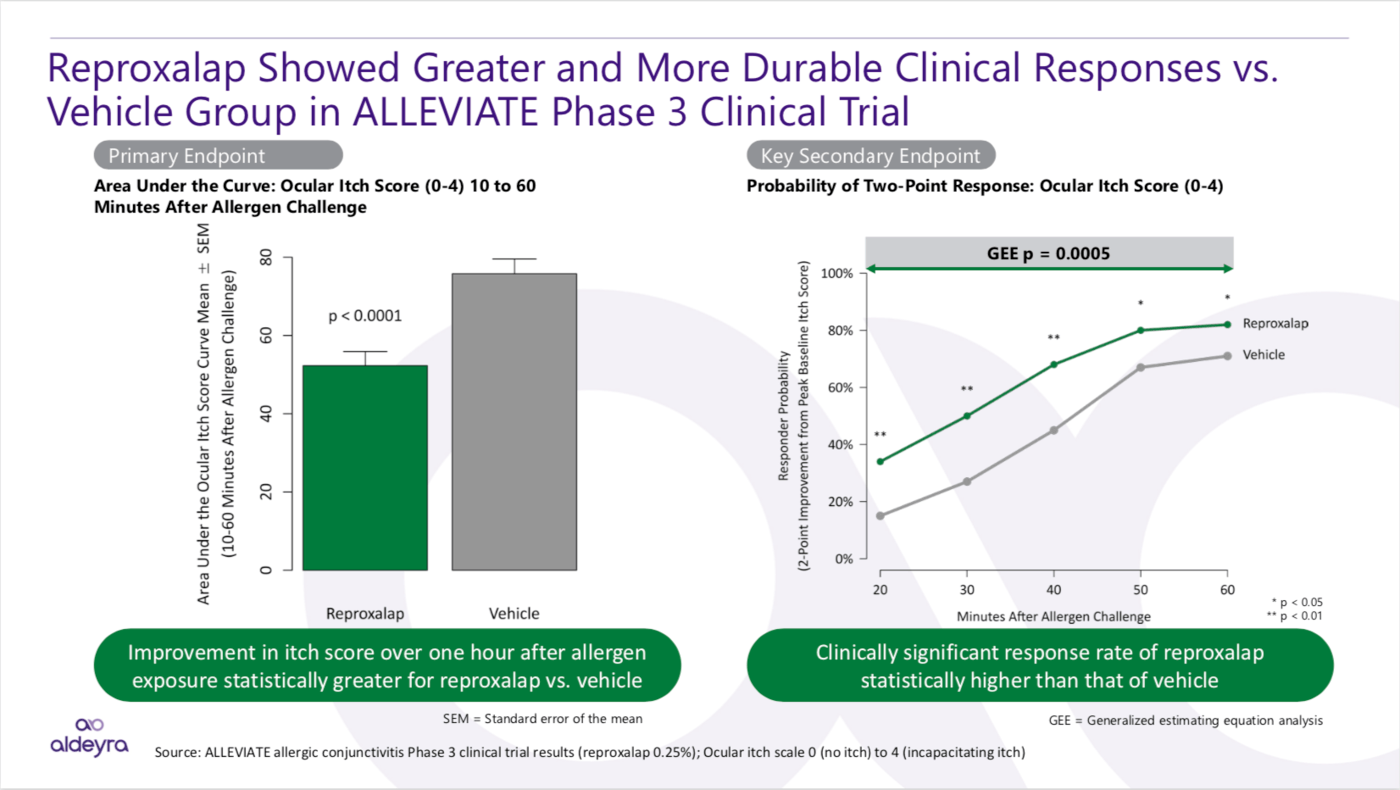
Clinical Results in Allergic Conjunctivitis
In the Phase 3 ALLEVIATE clinical trial in patients with allergic conjunctivitis, the primary and key secondary endpoints were achieved: reproxalap treatment led to ocular itch score reduction and clinically meaningful responses, respectively, in a manner that was statistically superior to placebo.