For the approximately 39 million patients in the United States with dry eye disease, the ocular pain, dryness, and gritty sensation are all too familiar and persistent symptoms of the disease. There are presently three FDA-approved products for the treatment of dry eye disease, however, a significant clinical unmet need remains. There is an opportunity for novel approaches to the treatment of Dry Eye Disease that can demonstrate improvements in the effectiveness of both the signs and symptoms of the disease as well as therapies with a more rapid onset of action.
There are approximately 39 million dry eye disease patients in the United States.
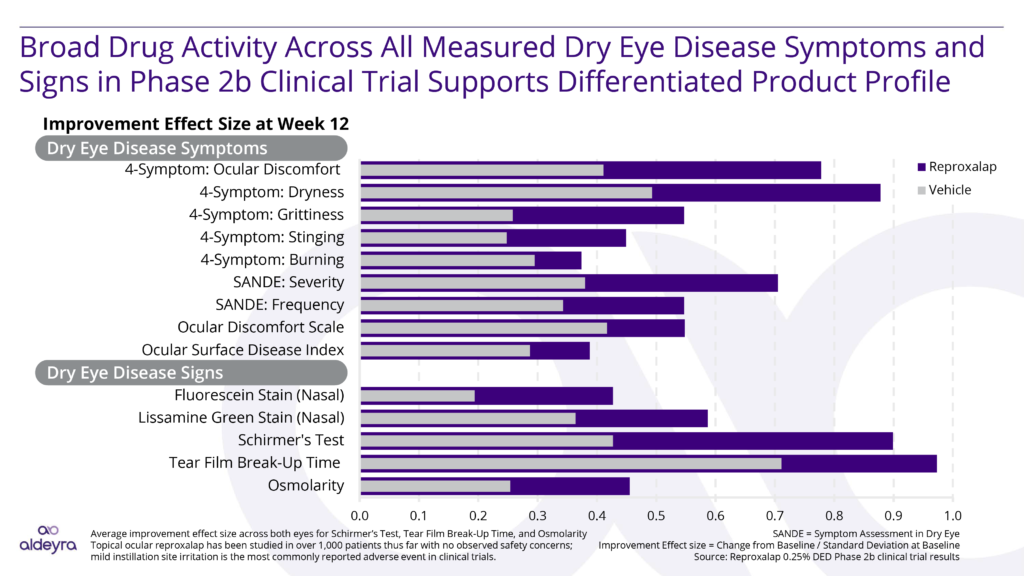
Reproxalap: Our Novel Small Molecule Drug Candidate for Dry Eye
By inhibiting RASP, which are elevated in a variety of inflammatory diseases, reproxalap represents a novel mechanism for diminishing ocular inflammation in dry eye disease. In a number of clinical trials, reproxalap demonstrated consistent statistically significant and clinically relevant activity. We believe that reproxalap may have a commercially differentiated product profile versus currently approved drugs for each indication, having shown the potential for early and broad activity in dry eye disease. Additionally, reproxalap, if approved, has the potential of being the only product that may be able to effectively treat dry eye disease, uniquely addressing the needs of the large underserved population that suffers from this disease. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 1,800 patients with no observed safety concerns; mild and transient instillation site discomfort is the most commonly reported adverse event in clinical trials.
Aldeyra intends to advance 0.25% topical ocular reproxalap, our lead RASP inhibitor, for the treatment of dry eye disease.