Proliferative Vitreoretinopathy
Proliferative vitreoretinopathy (PVR) is a rare inflammatory disorder of the retina that leads to severe retinal scarring and blindness and is the leading cause of failure of retinal reattachment surgery. Over 50% of PVR cases result in severe uncorrectable vision loss (visual acuity of 20/320 or worse), and 76% of PVR patients suffer from at least moderate uncorrectable vision loss. PVR occurs after up to 10% of surgeries for retinal detachment and 50% or more of surgeries for open globe injury. Based on the prevalence of primary retinal detachment, in addition to retinal detachment that occurs as a result of trauma, we estimate that there are, in aggregate, more than 20,000 treatable cases of PVR in the United States, Europe, and Japan. By inhibiting cell growth and thereby diminishing scar formation, ADX-2191 has the potential to be the first FDA-approved drug for prevention of PVR. In April 2018, ADX-2191 received orphan drug designation from the FDA for the prevention of PVR.
There are approximately more than 20,000 treatable cases of PVR in the United States, Europe, and Japan combined.
Clinical Results in Proliferative Vitreoretinopathy
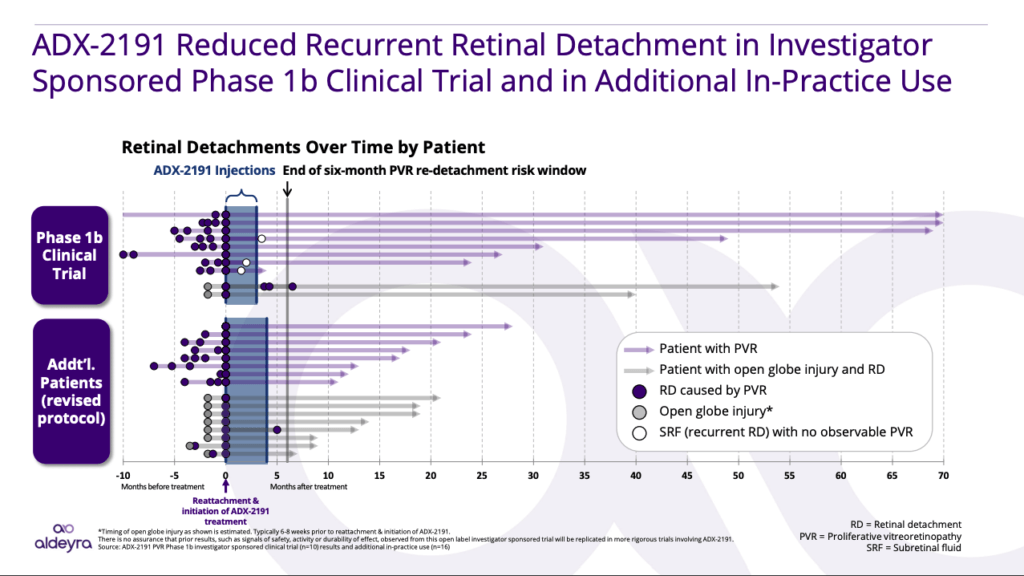
In an Investigator Sponsored Trial, led by Dr. Dean Eliott of the Massachusetts Eye and Ear Infirmary and Harvard Medical School, ADX-2191 led to rates of retinal detachment that were less than what would be expected from the standard of care (no treatment).